CDISC’s Requirements About SENDA Story by MedicilonSEND (Standard for Exchange of Nonclinical Data, Non-Clinical Data Interchange Standards Consortium)Clinical Data Interchange Standards Consortium (Clinical Data Interchange Standards Consortium) NumeSEND (Standard for Exchange of Nonclinical Data, Non-Clinical
Data Interchange Standards Consortium)Clinical Data Interchange Standards Consortium (Clinical
Data Interchange Standards Consortium) Numerical formalism. On September 15th of this year, the FDA
CDER issued an enforcement request IND and an NDA/BLA application, supporting
the submission of a non-payment test SEND number (Dataset). The Dataset code
for the data set is to be confirmed and sent by the FDA SEND formal communication request. Mr. Harahara, Director of FDA
standardization research. Origin: FDA When did it Start to Operate the SEND Dataset?
FDA CDER general request IND on
September 15, 2021, support NDA/BLA China-China demand negotiations, non-table
trial SEND Dataset, including comprehensive GLP and non-GLP trial. If the SEND
Dataset cannot be passed, the FDA approval has been rejected. Support for
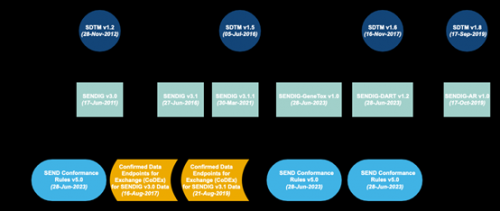
other research categories than SENDIG. For example, SENDIG-DART v1.2 has been
established, and DART research collection includes certain specific numerical
recommendations, especially embryo-fetal development (EFD) research, and
juvenile animal behavioral toxicity research. SENDIG-AR v1.0 Supports the basic
infrastructure and ongoing research. In addition to the model implementation
guidelines, we will develop the consistency guideline, so you can be sure of
the generative number structure combination.
Under what circumstances will FDA
refuse to accept a submitted SEND Dataset?
If the submitted
SEND Dataset has the following problems, FDA will refuse to accept it: • Missing corresponding ts.xpt Dataset • Incorrectly filling in the STF file tag of Dataset • Missing DM Dataset and define.xml • Missing STF files [1]. CDISC
Website: SEND. https://www.cdisc.org/standards/foundational/send © 2023 Medicilon |
StatsAuthorMedicilonCambridge, MAAboutMedicilon is an integrated contract research organization (CRO), providing comprehensive one-stop new drug R&D services for pharmaceutical enterprises and scientific research institutions around the w.. more..Writing
|



 Flag Writing
Flag Writing